Downstream Processing of Primary T-Cells Using Thermo Scientific™ General-Purpose Pro Centrifuges
Introduction
Washing and concentrating cells is one of the main steps in cell therapy research and manufacturing. During this step, expanded cell cultures are concentrated and washed in suitable buffer solutions to prepare for downstream steps, such as fill and formulation. The primary method for many years now has been batch centrifugation, although alternative methods such as continuous-flow centrifugation, acoustic-based separation, and tangential flow filtration have emerged over the years.
Here we show the advantages of batch centrifugation, as well as innovative solutions using Thermo Scientific™ General-Purpose Pro Centrifuges that can be optimized for downstream processing of primary T-cells. We also share some exemplary results for viability and recovery of T-cells after washing and concentration.
Fit-for-Purpose Equipment Features for Cell Therapy Manufacturing Applications
Secure and Total Control: SECURE-Spin
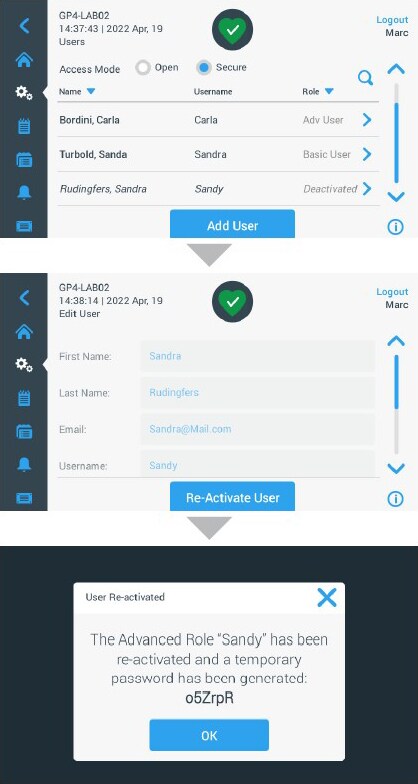
The centrifuges provide an advanced user management system in SECURE-Spin mode, whereby users are assigned different roles and privileges to maintain secure control, a feature which supports FDA 21 CFR Part 11 compliance (figure 1). The advanced functions of the SECURE-Spin mode allow you to:
- Register up to 120 individuals with unique user names and passwords
- Assign individual privileges and roles to each user, with different authorities
- Track all user changes to programs or settings with an audit trail
Reproducibility: The ACE Integrator Function
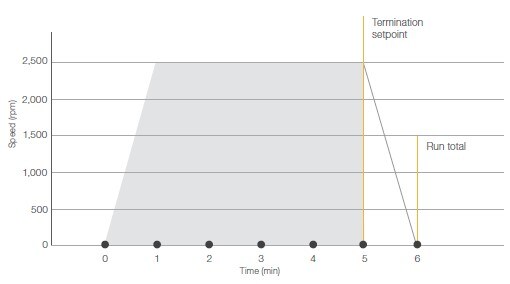
The Thermo Scientific™ Accumulated Centrifugal Effect™ (ACE™) Integrator Function is a mathematical model that helps to transfer applications and parameter settings between centrifuges by automatically modifying the run duration as a substitute of time (figure 2).
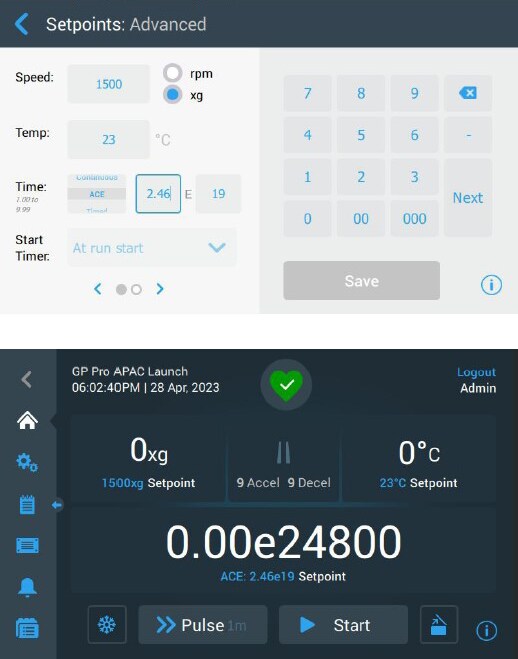
The ACE function can be enabled by navigating to “Settings” and then “Controls”, before changing the setpoint mode to “Advanced”. It has been engineered to help ensure different batches of sample receive the same desired centrifugal effect, regardless of the weight of the sample, equipment age, or environmental factors such as altitude (figure 3). Benefits of function activation for General-Purpose Pro Centrifuges include:
- Helps enable the same total accumulated centrifugal force regardless of conditions (e.g., time of run, rotor loading, and sample load)
- Gives reproducible yields by automatically compensating for variations in acceleration by adjusting the centrifuge run time
- Supports vital reproducibility in cell and gene therapy
To determin the ACE value, a working protocol is needed in which speed and run time are known. In ACE mode, enter the optimal speed value and a “high” ACE value (the higher value is to ensure that the run is not stopped prematurely). Start a stopwatch at the start of the centrifugation run, stop the centrifuge when the time on the stopwatch has reached the known run time, and record the ACE value shown on the screen.
Contamination Prevention: ClickSeal™
Thermo Scientific™ ClickSeal™ Biocontainment Lids protect samples from contaminating each other, enabling centrifugation of different samples in the same run. In the case of any spillage events the sample is contained within its bucket, thus protecting samples in other buckets, users, and laboratory environment. Key features of ClickSeal Lids are:
- Ergonomic design for easy opening and closing using only one hand, either left-handed or right-handed
- Biocontainment sealing options for glove-friendly use
- Simple operation for all laboratory users, with the elimination of multi-turn screw caps and complicated high-pressure clips
- Biocontainment certification by the National Institute for Health Protection, Porton Down, UK
Health status in cell therapy research and manufacturing, particularly for autologous cell types, is not readily available, and promptness is key for success. Thus, laboratory equipment must be ready to function flawlessly when needed. The “Health Status” button on our centrifuges tracks and records each alarm, alert notification, and rotor life cycle (figure 4). This prevents downtime, which is vital for process continuity.
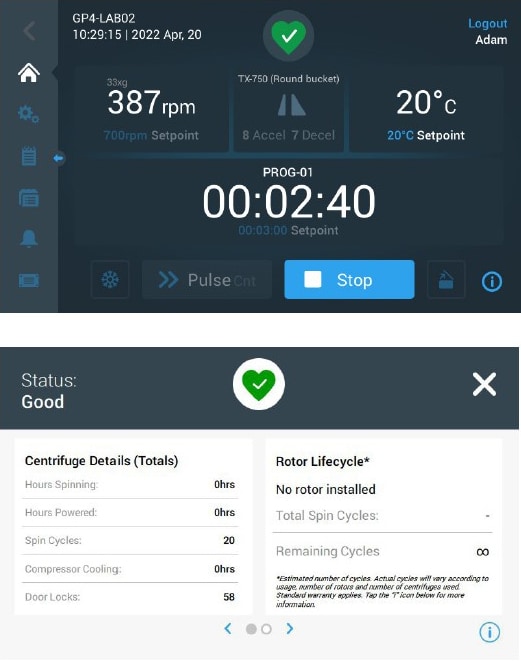
Rotor Management: SMART-Spin™
The SMART-Spin™ Advanced Rotor Management System maximizes acceleration, deceleration, and residual load imbalance for each rotor and bucket option. General-Purpose Pro Centrifuges further enable fine-tuning of centrifugation parameters with high precision (increments of 1rpm for speed and 1s for time). Furthermore, gentle acceleration and deceleration behaviors help maximize pellet integrity while keeping up process efficiency. In the example below, we employed acceleration profile 9 and deceleration profile 7 to minimize cell loss and maintain viability.
Viability and Recovery of T-Cells
Methods
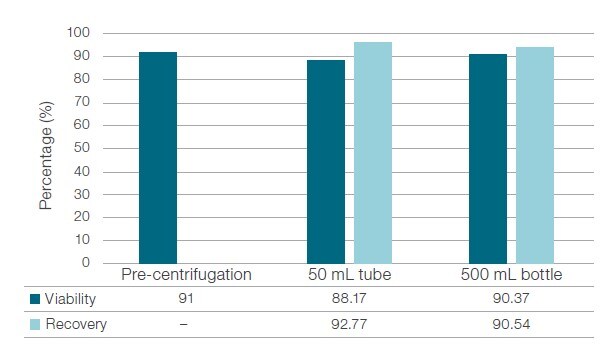
Primary T-cells provided by healthy donors were transferred into 50mL and 500mL conical containers at a concentration of 1 x 106 cells/mL. Cell counting was performed using the Via2-Cassette™ device and NucleoCounter™ NC-200™ instrument (ChemoMetec; Allerod, Denmark). Containers were centrifuged in the Thermo Scientific™ Multifuge™ X4R Pro General-Purpose Centrifuge at 400 x g at room temperature for 10 minutes. Viability and recovery of T-cells were determined after washing and concentration. Note: The cell concentration of 1 x 10⁶ cells/mL used is four-fold to five-fold lower than usual values, in order to simulate a worst-case scenario.
Results
Our results suggested that both conical container types, 50mL and 500mL, performed equivalently in concentrating T-cells, with a minimal decrease in viability and high recovery rates (figure 5).
Conclusions
Batch centrifugation has been the workhorse of cell therapy manufacturing for decades. Based on our findings, we conclude it is the preferred method because of the following advantages:
- No limitation on applicable cell types
- No clogging when high cell densities are processed
- High performance with low cell density
- Active control and maintenance of process temperature
- Low capital investment, with low cost of maintenance and operation
- Uncomplicated process development
- Flexible enough to be used for numerous laboratory applications



