CAR T-Cell Therapy Solutions
 Sample Collection & Cell Isolation
Sample Collection & Cell Isolation
Leverage magnetic beads and reagents, trusted solutions for sample collection and T-cell isolation.
 Cell Modification
Cell Modification
Find tools and reagents needed for T-cell engineering and manipulation.
 Cell Culture & Expansion
Cell Culture & Expansion
Benefit from trusted products for cell culture, activation and expansion of T-cells including media, reagents and equipment.
 Cell Analysis & Cryopreservation
Cell Analysis & Cryopreservation
Explore reliable and high-quality solutions for cell analysis and cryopreservation.
 Bioprocessing
Bioprocessing
Discover scalable solutions for advancing cell therapy process.
 Safety
Safety
Complete your order with safety products.
View relevant resources
Related Resources
All you need to know about
Focus on CAR T-cells
CAR T-cell therapy is an innovative development in the field of oncology, particularly for patients with late-stage or rare forms of cancer. We offer products and tools to help researchers and manufacturers develop cell therapies. Here you find solutions that support you across the entire workflow, from T-cell isolation and activation to cell engineering and expansion, including scalable solutions to help get you from basic research to the clinic.
Find out more
Frequently Asked Questions
What are CAR T-cells?
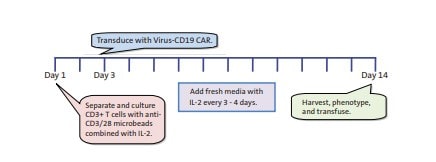
Chimeric antigen receptor T-cells (CAR T-cells) are made from a patient's T-cells that are harvested through apheresis and genetically modified in a laboratory to express a chimeric antigen receptor. When the CAR T-cells are reinjected into the patient's body, they target cell-surface antigens expressed on tumour cells and eliminate cancer cells throughout the body.
Can CAR T-cells apply to all cancer treatments?
CAR T therapy has been shown to achieve complete remission in patients with liquid cancers like B-cell malignancies. However, CAR T therapy has not had similar success targeting solid tumours. Solid cancerous masses are often hypoxic, acidic, and immunosuppressive, making it difficult for T-cells to infiltrate and survive in that environment.
What are autologous or allogeneic CAR T-cell therapies?
Autologous CAR T-cell therapies use a patient's own immune cells while allogeneic, or off-the-shelf, CAR T-cell products derive from healthy blood donors. Autologous CAR T-cells make up most clinically proven CAR cell products and are part of the standard of care for multiple blood diseases. Despite its effectiveness this strategy has several limitations in terms of costs and logistics that could be overcome by allogeneic CAR T-cell therapy. Many companies and start-ups were focussing on allogeneic CAR T therapies, as allogeneic immunotherapies were seen as the next big thing in cell therapies. However, allogeneic CAR cell products come with their own challenges, including the potential to induce graft-versus-host-disease and other uncertainties like chromosomal abnormality.
What are the main challenges of CAR T-cell manufacturing?
The main challenges of CAR T-cell manufacturing include supply constraints, scalability, and storage issues.
What are the alternatives for the viral CAR T-cell manufacturing approach?
All of the FDA-approved CAR T-cell therapies rely on a disarmed virus to generate CAR T-cells. However, dealing with virus-derived vectors brings potential obstacles in the CAR T-cell manufacturing process due to strict regulations, speed of manufacturing, toxicities and high costs. To overcome these hurdles, currently several research teams have used gene-editing technologies like TALON and CRISPR to induce the donated T-cells to produce CARs.
Need More Information or Help?
Contact us

Talk to a Sales Representative
If you have questions regarding to our products, services and offers, or should you require technical assistance, please request a call-back to be contacted by our Specialist Sales Representative!

Talk to a Safety Specialist
Contact us today to talk about any safety issues or to advise on product selection.

Fisher Scientific Biotech News
Join our mailing list and receive updates on new product announcements, special promotions, sales and more.













22_04.jpg-650.jpg)